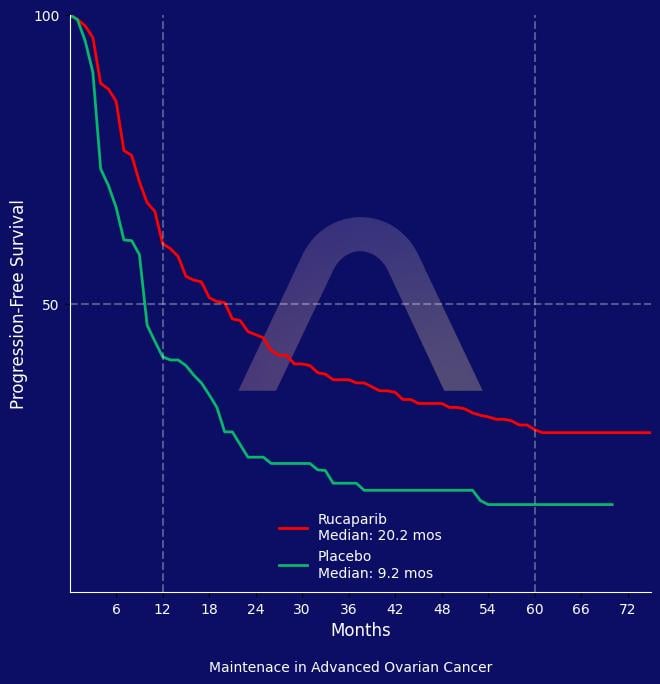
The ATHENA-MONO study demonstrated significant improvement in progression-free survival with rucaparib as maintenance therapy for newly diagnosed advanced ovarian cancer. The hazard ratio favored rucaparib in both homologous recombination-deficient and intent-to-treat populations. The safety profile of rucaparib was manageable, with common adverse events including anemia and liver enzyme elevations.
Study
|
Randomized, double-blind, placebo-controlled, phase III study [ATHENA-MONO/GOG-3020/ENGOT-ov45/NCT03522246] |
| Newly diagnosed stage III-IV ovarian cancer who responded to platinum doublet chemotherapy |
| Rucaparib maintenance (n=427) vs Placebo (n=111)
|
Efficacy
|
mPFS: 20.2 mos vs 9.2 mos (HR 0.53 [0.42-0.69]) in ITT |
| mPFS: 31.4 mos vs 12.0 mos (HR 0.52 [0.35-0.76]) in HRD |
| OS in ITT: NR vs 46.2 mos (HR 0.83 [0.58-1.17])
|
Safety
|
Grade >=3 AEs: anemia (28.7% vs 0%), neutropenia (14.6% vs 0.9%), ALT/AST increase (10.6% vs 0.9%) |
| Discontinuations due to AEs: 11.8% vs 5.5%
|
Ann Oncol. Published online XXX
http://doi.org/10.1016/j.annonc.2025.10.007
Reviewed by Ulas D. Bayraktar, MD on Nov 17, 2025