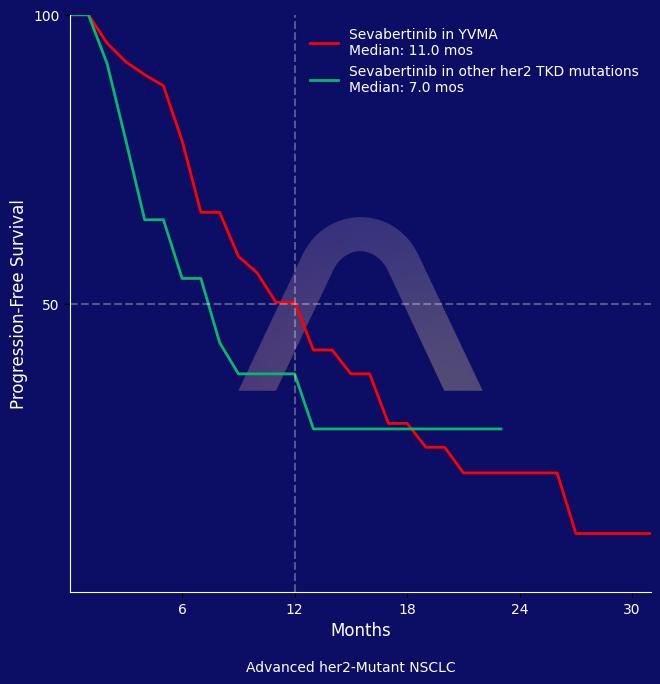
Sevabertinib showed significant activity in patients with HER2-mutant NSCLC, achieving high response rates across different cohorts. The drug demonstrated a favorable response even in those previously treated with HER2-directed therapies. Common side effects included diarrhea, with grade 3 events managed effectively, allowing most patients to continue treatment.
Study
|
Open-label, multicenter, multicohort, phase 1-2 study [SOHO-01] |
| Locally advanced or metastatic HER2-mutant NSCLC |
| Sevabertinib 20 mg twice daily (n=209) across three cohorts: D (n=81; no prior HER2-directed therapy); E (n=55; prior HER2-directed ADCs); F (n=73; no prior systemic therapy)
|
Efficacy
|
ORR: 64% vs 38% vs 71% (Cohorts D vs. E vs. F) |
| mDOR: 9.2 mos vs 8.5 mos vs 11.0 mos |
| mPFS: 8.3 mos vs 5.5 mos vs NE
|
Safety
|
Grade >=3 AEs: 31% vs 31% vs 23% |
| Diarrhea (86% vs 91% vs 84%), Grade 3 (23% vs 11% vs 5%) |
| Treatment discontinuation due to AEs: 5% vs 4% vs 1%
|
N Engl J Med 2025;393:1819-32
Le X, Kim TM, Loong HH New Drug: Sevabertinib in Advanced HER2-Mutant NSCLC
http://doi.org/10.1056/NEJMoa2511065
Reviewed by Ulas D. Bayraktar, MD on Nov 17, 2025