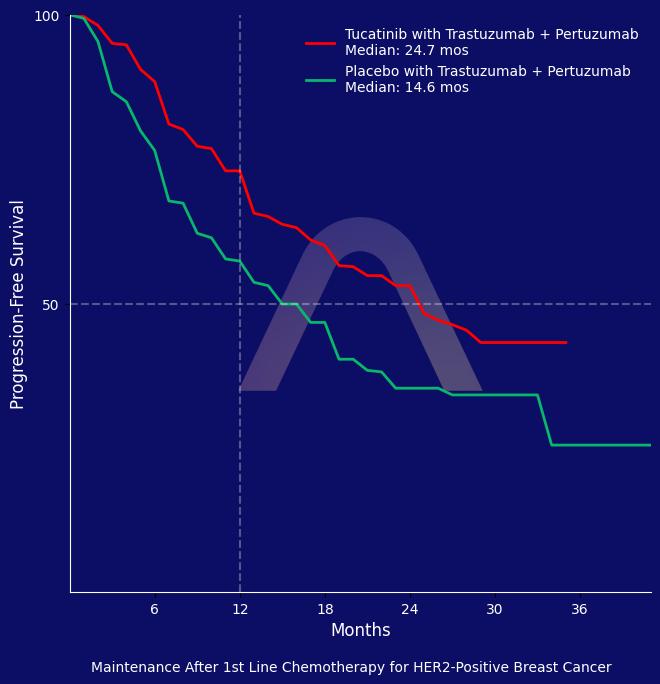
The study found that adding tucatinib to trastuzumab and pertuzumab significantly improved progression-free survival compared with placebo, with a hazard ratio of 0.641. Tucatinib showed a consistent benefit across patient subgroups, including those with brain metastases. Despite the efficacy, treatment-emergent adverse events were higher in the tucatinib arm, with a notable increase in elevated liver enzymes.
Study
|
Randomized, double-blind, placebo-controlled, international, phase III trial [HER2CLIMB-05] |
| First-line maintenance therapy for HER2-positive metastatic breast cancer after taxane-trastuzumab-pertuzumab |
| Tucatinib (n=326) vs placebo (n=328) in combination with trastuzumab and pertuzumab
|
Efficacy
|
mPFS: 24.9 mos vs 16.3 mos (tucatinib vs. placebo) (HR 0.641 [0.514-0.799])
|
Safety
|
Grade >=3 TEAEs: diarrhea (6.1% vs 4.0%), elevated ALT (13.5% vs 0.6%), elevated AST (7.1% vs 0.6%) |
| Serious TEAEs: 16.9% vs 8.0% |
| Treatment discontinuation due to TEAEs: 13.5% vs 4.6%
|
J Clin Oncol. Published online December 10, 2025
http://doi.org/10.1200/JCO-25-02600
Reviewed by Ulas D. Bayraktar, MD on Jan 21, 2026