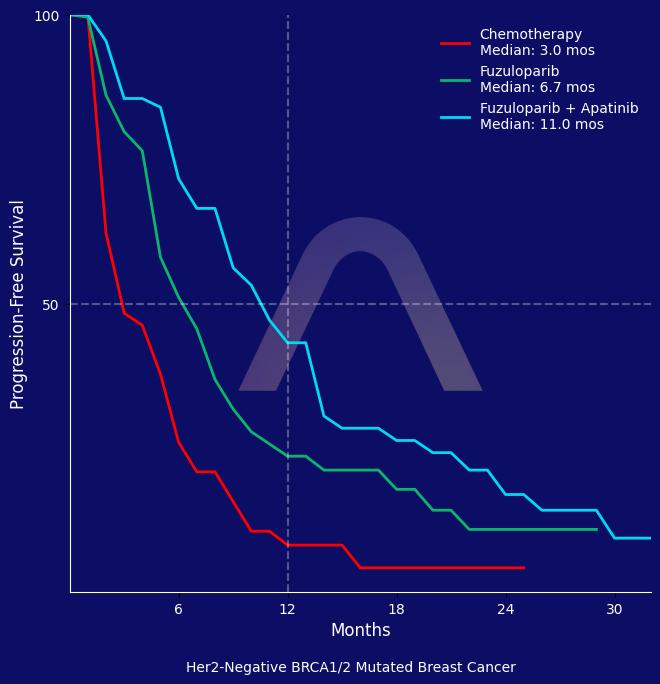
The interim analysis of the FABULOUS study showed that fuzuloparib combined with apatinib significantly improved progression-free survival compared with chemotherapy in patients with HER2-negative metastatic breast cancer with germline BRCA1/2 mutations. Fuzuloparib monotherapy also demonstrated a benefit over chemotherapy. Both treatment regimens had acceptable safety profiles.
Study
|
Multicentre, three-arm, open-label, randomised, phase 3 trial [FABULOUS] |
| HER2-negative metastatic breast cancer with germline BRCA1/2 mutations |
| Fuzuloparib+apatinib (n=70) vs Fuzuloparib (n=67) vs Chemotherapy (n=66)
|
Efficacy
|
mPFS: 11.0 mos vs 6.7 mos vs 3.0 mos (FA vs. F alone vs. chemo) (HR F+A vs C 0.27 [0.17-0.43]; HR F vs C 0.49; HR F+A vs F 0.60 [0.4-0.91])
|
Safety
|
Grade >=3 AEs: decreased neutrophil count (13% vs 21% vs 24%), anaemia (11% vs 37% vs 39%), hypertension (13% vs 0% vs 2%) |
| Serious AEs: 13% vs 18% vs 14% |
| Discontinuations: 1% vs 1% vs 3%
|
Lancet Oncol 2025;26:1563-1574
Li H, Liu J, Ouyang Q New Protocol: Fuzuloparib with Apatinib for BRCA1/2 Mutated Breast Cancer
http://doi.org/10.1016/S1470-2045(25)00523-6
Reviewed by Ulas D. Bayraktar, MD on Dec 8, 2025