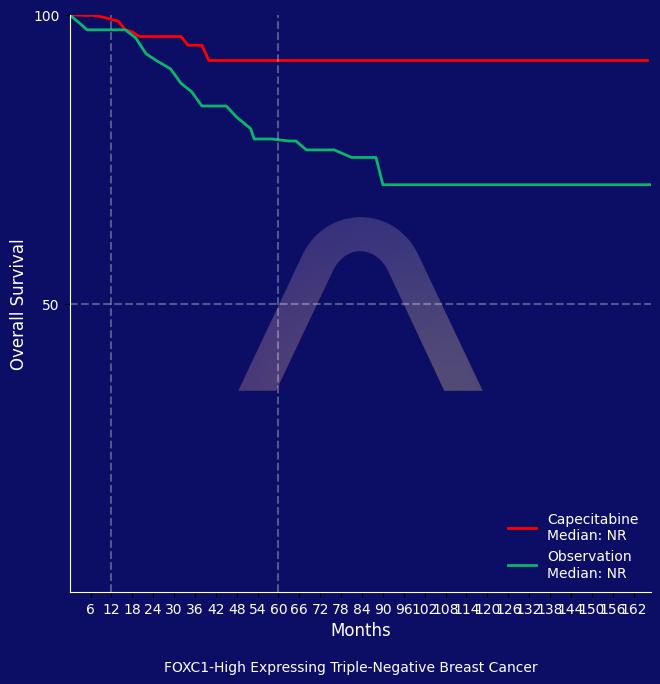
The SYSUCC-001 trial showed that metronomic capecitabine as extended adjuvant therapy significantly improves 10-year disease-free survival in early triple-negative breast cancer. Although the difference in 10-year overall survival was not statistically significant, the treatment offered a marked disease-free survival advantage. FOXC1 expression may identify patients who particularly benefit from this approach.
Study
|
Randomized, open-label, multicenter, phase 3 trial [SYSUCC-001] |
| Women with stage T1b-3 N0-3c M0 triple-negative breast cancer post standard adjuvant therapy |
| Metronomic capecitabine (n=221) vs observation (n=213) for 1 year
|
Efficacy
|
10-yr DFS: 78.1% vs 66.6% (capecitabine vs. observation) (HR 0.61 [0.43-0.88]) |
| 10-yr OS: 82.4% vs 73.7% (HR 0.67 [0.45-1.01]) |
| 10-yr distant-DFS: 78.1% vs 66.5% (HR 0.61 [0.43-0.88]) |
| 10-yr LRFS: 81.6% vs 69.9% (HR 0.60 [0.40-0.89]) |
| Among pts with high FOXC1 expression, DFS and OS were significantly longer in capecitabine arm, whereas among pts with low FOXC1 expression, there was none.
|
Safety
|
Safety data not reported in this article
|
Lancet Oncol 2025;26:1575-1583
Yuan J, Bi XW, Hua X New Reference: Adjuvant Capecitabine for Triple-Negative Breast Cancer
http://doi.org/10.1016/S1470-2045(23)00398-7
Reviewed by Ulas D. Bayraktar, MD on Dec 8, 2025