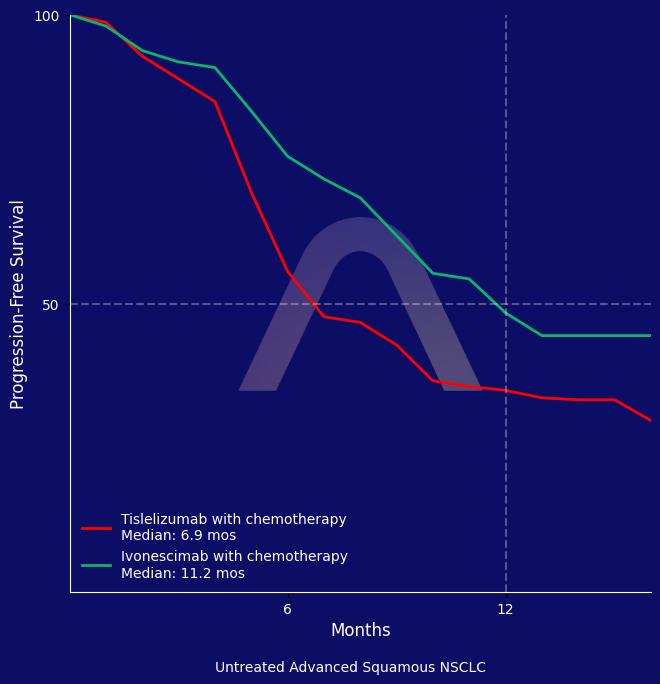
The HARMONi-6 trial showed that ivonescimab combined with chemotherapy significantly improved progression-free survival compared to tislelizumab combined with chemotherapy. The treatment demonstrated a higher response rate and a manageable safety profile, highlighting the role of anti-VEGF therapy in the treatment of squamous NSCLC.
Study
|
Randomised, double-blind, phase 3 trial [HARMONi-6] |
| Advanced squamous non-small-cell lung cancer, untreated Stage IIIB/IIIC or IV |
| Ivonescimab (n=266) plus chemotherapy vs tislelizumab (n=266) plus chemotherapy
|
Efficacy
|
mPFS: 11.1 mos vs 6.9 mos (Ivonescimab vs. Tislelizumab)(HR 0.60 [0.46-0.78]) |
| Objective response rate: 76% vs 67%
|
Safety
|
Grade >=3 AEs: neutrophil count decreased (32% vs 26%), white blood cell count decreased (11% vs 9%), anaemia (6% vs 4%) |
| Grade >=3 immune-related AEs: 9% vs 10% |
| Haemorrhage: 2% vs 1% |
| Treatment discontinuation due to AEs: 3% vs 4%
|
Lancet Published online October 19, 2025
http://doi.org/10.1016/S0140-6736(25)01848-3
Reviewed by Ulas D. Bayraktar, MD on Nov 15, 2025