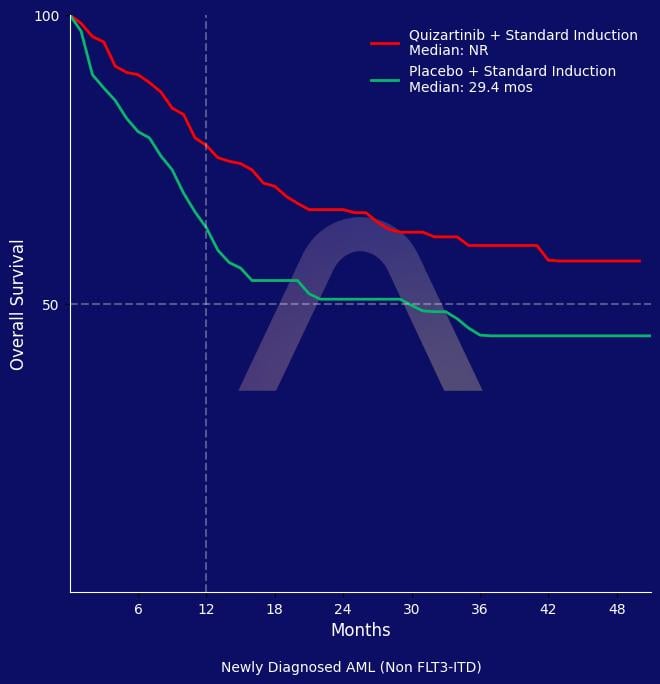
The QUIWI study demonstrated that quizartinib, when added to standard chemotherapy, significantly prolonged both event-free and overall survival in patients with newly diagnosed FLT3-ITD-negative acute myeloid leukemia. The study found that the three-year overall survival rate was higher with quizartinib than with placebo. Quizartinib was well tolerated with manageable safety concerns.
Study
|
Randomized, double-blind, placebo-controlled, phase 2 study [QUIWI] |
| Newly diagnosed FLT3-ITD-negative acute myeloid leukemia patients aged 18-70 |
| Quizartinib 60 mg daily (n=180) vs placebo (n=93) combined with standard chemotherapy
|
Efficacy
|
mEFS: 20.4 mos vs 9.9 mos (HR 0.72 [0.53-1.00], p=0.046) |
| mOS: Not reached vs 29.3 mos (HR 0.63 [0.44-0.91], p=0.012) |
| 3-yr OS rates: 60.8% vs 45.7%
|
Safety
|
Grade >=3 AE: QTc prolongation (3.4% vs 2.2%) |
| Serious AEs: 56.2% vs 57.6% |
| Treatment discontinuation due to AEs: 14.6% vs 10.9%
|
J Clin Oncol. Published online 2025 October 13.
http://doi.org/10.1200/JCO-25-01841
Reviewed by Ulas D. Bayraktar, MD on Oct 23, 2025