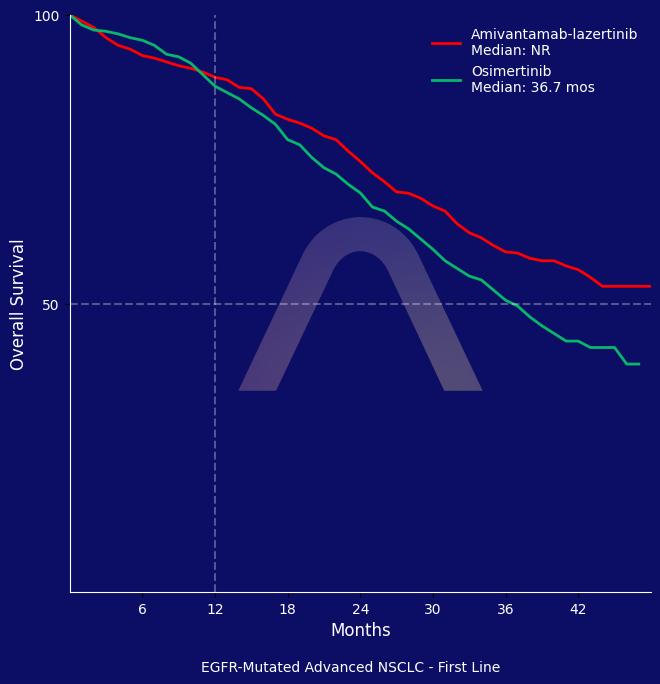
The MARIPOSA trial demonstrated that amivantamab-lazertinib significantly improved overall survival compared to osimertinib in EGFR-mutated advanced NSCLC in 1st line setting, with a 25% reduction in the risk of death. However, amivantamab-lazertinib was associated with a higher incidence of grade 3 or higher adverse events, particularly venous thromboembolism and infusion-related reactions.
Study
| Randomized, international, phase 3 trial [MARIPOSA, NCT04487080] |
| Previously untreated EGFR-mutated (exon 19 deletion or L858R substitution) advanced NSCLC |
| Amivantamab–Lazertinib (n=429) vs Osimertinib (n=429) vs Lazertinib (not summarized) |
Efficacy
| 3-yr OS: 60% vs 51% |
| mOS: NE vs 36.7 mos (HR 0.75 [0.61-0.92]) |
| Median time to first subsequent therapy: 30.3 mos vs 24.0 mos (HR 0.76 [0.64-0.90]) |
| Intracranial PFS: 25.4 mos vs 22.2 mos (HR 0.79 [0.61-1.02]) |
Safety
| Grade >=3 AEs: 80% vs 52% |
| Serious AEs: 55% vs 41% |
| Venous thromboembolism: 40% vs 11% |
| Infusion-related reaction: 65% vs 0% |
N Engl J Med. Published online September 7, 2025.
http://doi.org/10.1056/NEJMoa2503001
Reviewed by Ulas D. Bayraktar, MD on Oct 13, 2025
- Chemotherapy Protocols
- Summarized References
- Oncology Drug Specs
- TNM Staging
- BSA, CrCl Calculator