Study
| Randomized, controlled, phase III study [NeoADAURA] NCT04351555 |
| Resectable, EGFR-mutated, stage II-IIIB NSCLC |
| Osimertinib+chemotherapy (n=121) vs Osimertinib (n=117) vs Placebo+chemotherapy (n=120) |
Efficacy
| MPR: 26% vs 25% vs 2% |
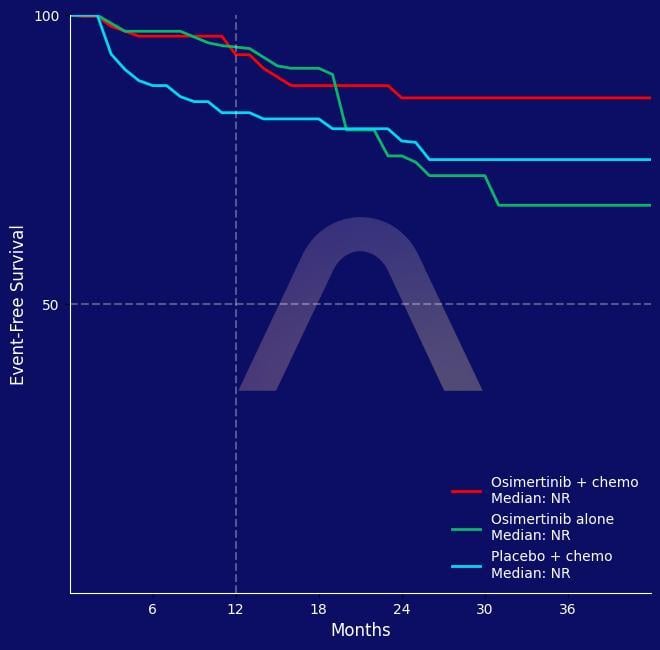
| EFS at 12mos: 93% vs 95% vs 83% |
Safety
| Grade >=3 AEs: 36% vs 13% vs 33% |
| Serious AEs: 20% vs 11% vs 20% |
| Discontinuations: 2% vs 2% vs 2% |
J Clin Oncol Published online June 2, 2025
He J,Tsuboi M,Weder W Neoadjuvant Osimertinib for Resectable EGFR-Mutated Non Small Cell Lung Cancer
http://doi.org/10.1200/JCO-25-00883
Reviewed by Elvin Chalabiyev, MD on Sep 3, 2025